6. Biomolecules - part 05 - Enzymes
- Get link
- X
- Other Apps
6. Biomolecules - part 05 - Enzymes
Enzymes :
- Thousands of different chemical reactions take place automatically at a given time in a tiny living cell.
- The reactions take place at the body temperature.
- If these enzymes were not present in the cell, either the reactions would not occur or if they occur they would occur at a very very slow rate.
- German chemist Edward Buchner discovered enzymes by accident.
- Buchner discovered that living cells were not necessary but that yeast extract could bring about fermentation.
- He then coined the term Enzyme (Gk. En = in, zyma = yeast i.e. in yeast). This term is now commonly used for all biocatalysts.
- Each enzyme catalyzes a small number of reactions, specifically perhaps only one.
- Substrate : The substance upon which an enzyme acts is termed as the substrate.
- Endo-enzymes : The enzymes which act within the cell in which they are synthesizedare known as endo-enzymes e.g., enzymes produced in the chloroplast and mitochondria
- Exo-enzymes : If they act outside the cell in which they are synthesized, they are known as exo-enzymes e.g., enzymes released by many fungi.
- These enzymes, synthesised by living cell, retain their catalytic property even when extracted from cells.
- Rennet tablets used for coagulating milk protein casein (cheese) contain renin enzyme that is obtained from the stomach of calf.
- On the basis of chemical composition, enzymes can be put into two categories.
- Purely proteinaceous enzymes e.g. proteases that spilt protein
- Conjugated enzymes are made up of a protein to which a non-protein prosthetic group is attached.
- The prosthetic group is firmly bound to the protein component by chemical bonds and is not removed by hydrolysis.
- If the prosthetic group is removed the protein part of the enzyme becomes inactive.
- There are enzymes which require certain organic compounds and inorganic ions for their activity.
- Co-enzymes : The organic compounds that are tightly attached to the protein part are called coenzymes
- Co-factors : the inorganic ions which are loosely attached to the protein part are called co-factors.
- Some of the organic co-enzymes are -
- nicotinamide-adenine-dinucleotide (NAD) and
- flavin mononucleotide (FMN).
- Inorganic ions of metals which act as co-factors include magnesium, copper, zinc, iron, manganese etc.
- Iron (Fe++) is a co-factor of enzyme catalase, manganese is a co-factor of peptidases.
- Often metal co-factors are referred to as enzyme activators.
Proteinaceous Nature :
- All enzymes are basically made up of protein.
- All enzymes have specific 3-dimensional conformation.
- They have one or more active sites to which substrate (reactant) combines.
- The points of active site where the substrate joins with the enzyme is called substratebinding site.
- Enzymes are like inorganic catalysts and influence the speed of biochemical reactions but themselves remain unchanged.
- After completion of the reaction and release of the product they remain active to catalyse again.
- A small quantity of enzymes can catalyse the transformation of a very large quantity of the substrate into an end product.
- For example, sucrase can hydrolyse 100000 times of sucrose as compared with its own weight.
- The ability of an enzyme to catalyse one specific reaction and essentially no other is perhaps its most significant property.
- Each enzyme acts upon a specific substrate or a specific group of substrates.
- Enzymes are very sensitive to temperature and pH.
- Each enzyme exhibits its highest activity at a specific pH, called optimum pH.
- Any increase or decrease in pH causes decline in enzyme activity e.g. enzyme pepsin (secreted in stomach) shows highest activity at an optimum pH of 2 (acidic).
- Trypsin (in duodenum) is most active at an optimum pH of 9.5 (alkaline).
- Both these enzymes viz. pepsin and trypsin are protein digesting enzymes.
- Enzymes are destroyed at higher temperature of 60-70°C or below, they are not destroyed but become inactive.
- This inactive state is temporary and the enzyme can become active at suitable temperature.
- Most of the enzymes work at an optimum temperature between 20°C and 35°C.
- There are various ways of naming enzymes.
- Enzymes are named by adding the suffix-‘ase’ to the name of the substrate on which they act e.g. protease, sucrase, nuclease etc. which break up proteins, sucrose and nucleic acids respectively.
- The enzymes can be named according to the type of function they perform e.g. dehydrogenase remove hydrogen, carboxylase add CO; decarboxylases remove CO2, oxidases helping in oxidation.
- Some enzymes are named according to the source from which they are obtained e.g. papain from papaya, bromelain from the member of Bromeliaceae family, pineapple.
- According to international code of enzyme nomenclature, the name of each enzyme ends with an -ase and consists of double name.
- The first name indicates the nature of substrate upon which the enzyme acts and the second name indicates the reaction catalysed e.g. pyruvic decarboxylase catalyses the removal of CO2 from the substrate pyruvic acid.
- Similarly, the enzyme glutamate pyruvate transaminase catalyses the transfer of an amino group from the substrate glutamate to another substrate pyruvate.
Oxidoreductases :
- These are enzymes catalyzing oxidation and reduction reactions by the transfer of hydrogen and/or oxygen.
- e.g. alcohol dehydrogenase
- These enzymes catalyse the transfer of certain groups between two molecules.
- e.g. glucokinase
- These are enzymes catalyse hydrolytic reactions.
- This class includes amylases, proteases, lipases etc.
- eg. Sucrase
- These enzymes are involved in elimination reactions resulting in the removal of a group of atoms from substrate molecule to leave a double bond.
- It includes aldolases, decarboxylases, and dehydratases,
- e.g fumarate hydratase.
- These enzymes catalyze structural rearrangements within a molecule.
- Their nomenclature is based on the type of isomerism. Thus these enzymes are identified as racemases, epimerases, isomerases, mutases,
- e.g. xylose isomerase.
- These are the enzymes which catalyse the covalent linkage of the molecules utilizing the energy obtained from hydrolysis of an energy-rich compound like ATP, GTP
- e.g. glutathione synthetase, Pyruvate carboxylase.
- The basic mechanism by which enzymes catalyze chemical reactions begins with the binding of the substrate (or substrates) to the active site on the enzyme.
- The active site is the specific region of the enzyme which combines with the substrate.
- The binding of the substrate to the enzyme causes changes in the distribution of electrons in the chemical bonds of the substrate and ultimately causes the reactions that lead to the formation of products.
- The products are released from the enzyme surface to regenerate the enzyme for another reaction cycle.
- There are two models to explain the mechanism of forming Enzyme-Substrate complex :-
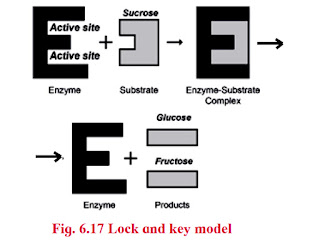
- Lock and Key model:
- Induced Fit model (Flexible Model)
- The specific action of an enzyme with a single substrate can be explained using a Lock and Key analogy first postulated in 1894 by Emil Fischer.
- In this analogy, the lock is the enzyme and the key is the substrate.
- Only the correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
Induced Fit model (Flexible Model):
- Koshland (1959) proposed the induced fit theory, which states that approach of a substrate induces a conformational change in the enzyme.
- It is the more accepted model to understand mode of action of enzyme.
- Unlike the lock-and-key model, the induced fit model shows that enzymes are rather flexible structures in which the active site continually reshapes by its interactions with the substrate until the time the substrate is completely bound to it (it is also the point at which the final form and shape of the enzyme is determined).
Factors Affecting Enzyme Activity :
Following factors affect enzyme activity :
1. Concentration of Substrate :
- Increase in the substrate concentration gradually increases the velocity of enzyme activity within the limited range of substrate levels.
- A rectangular hyperbola is obtained when velocity is plotted against the substrate concentration.
- Three distinct phases (A, B and C) of the reaction are observed in the graph.Where V = Measured velocity, Vmax = Maximum velocity, S = Substrate concentration, Km = Michaelis-Menten constant.
Km or the Michaelis-Menten constant :
- Defination :The substrate concentration (expressed in moles/lit) to produce half of maximum velocity in an enzyme catalysed reaction.
- It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value.
- Km value is a constant and a characteristic feature of a given enzyme.
- It is a representative for measuring the strength of ES complex.
- A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them.
- For majority of enzymes, the Km values are in the range of 10-5 to 10-2 moles.
2. Enzyme Concentration :
- The rate of an enzymatic reaction is directly proportional to the concentration of the substrate.
- The rate of reaction is also directly proportional to the square root of the concentration of enzymes.
- It means that the rate of reaction also increases with the increasing concentration of enzyme.
- The rate of reaction can also decreased by decreasing the concentration of enzyme.
3. Temperature :
- The enzymatic reaction occurs best at or around 37 degree Celsius which is the average normal body temperature in homeotherms.
- The rate chemical reaction is increased by a rise in temperature but this is true only over a limited range of temperature.
- Enzymes rapidly denature at temperature above 40oC.
- The activity of enzymes is reduced at low temperature.
- The temperature at which the enzymes show maximum activity is called Optimum temperature.
4. Effect of pH :
- Similar to temperature, there is also pH at which an enzyme will catalyze the reaction at the maximum rate.
- Every enzyme has different optimum pH value.
- The enzyme cannot perform its function beyond the range of its pH value.
5. Other Substances :
- The enzymes action is also increased or decreased in the presence of some other substances such as co-enzymes, activators and inhibitors.
- Most of the enzymes are combination of a co-enzyme and an apo-enzyme.
- Activators are the inorganic substances which increase the enzyme activity.
- Inhibitor is the substance which reduces the enzyme activity.
- Get link
- X
- Other Apps












Comments
Post a Comment